Emerging Innovations in Gadolinium-Based Imaging and Therapy
| Received 30 Mar, 2025 |
Accepted 31 Jul, 2025 |
Published 30 Sep, 2025 |
Gadolinium, a 64-atomic-number rare-earth lanthanide metal, is crucial in modern diagnostic medicine due to its paramagnetic properties. Its strong magnetic properties speed up the relaxation of water protons in MRI, making Gadolinium-Based Contrast Agents (GBCAs) important for accurate and early disease diagnosis. Gadolinium has also found utility in nuclear medicine, particularly in neutron capture therapy (NCT) for certain cancers. It is also explored in advanced bioimaging techniques due to its luminescence when doped with ligands or nanostructures. However, the clinical application of gadolinium is not without risks, including the toxic nature of free Gd3+ ions and potential side effects, especially in patients with impaired renal function. A recent review focuses on improving the biocompatibility and safety of GBCAs by developing macromolecular carriers, nanoparticle formulations and smart ligands that release gadolinium only under specific physiological conditions. These innovations aim to optimize distribution, minimise toxicity, and broaden the scope of gadolinium’s biomedical applications.
| Copyright © 2025 Kumbhare et al. This is an open-access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |
INTRODUCTION
Gadolinium, a soft, silvery-white rare-earth metal, reacts with oxygen, and water, forming an oxide layer that tarnishes its surface. In moist air, it oxidises to gadolinium oxide, while in water, it forms hydroxides, and hydrogen gas1. The trivalent ionic form (Gd3+) in MRI studies is effective due to its seven unpaired electrons, enhancing picture contrast, and enabling better differentiation between normal, and diseased tissues2. Gadolinium, a 64-atomic rare earth element, is known for its exceptional magnetic properties, particularly in its trivalent ionic state (Gd3+), which contains seven unpaired electrons, making it highly paramagnetic3. Gadolinium’s use has expanded beyond imaging to include targeted therapy, radiation, and theranostics, a unique paradigm that combines therapeutic, and diagnostic modalities on one platform4.
Gadolinium-Based Contrast Agents (GBCAs) are crucial in diagnostic radiology, particularly in MRI, due to their ability to shorten the T1 relaxation time of water protons. These agents enhance signal intensity in T1-weighted images, allowing for clearer visualisation of soft tissues, the brain, the spinal cord, and vasculature. Gadolinium is also being explored in theranostics, integrating diagnostic imaging with targeted therapy. Gadolinium is incorporated into nanoplatforms for simultaneous imaging, and drug delivery, improving treatment accuracy, and patient outcomes in oncology, and beyond5.
Due to its inherent qualities, gadolinium is a perfect fit for this dual purpose. Neutron capture therapy (NCT) is one of its main theranostic uses6. When neutrons are captured, the isotope gadolinium-157, which has a very high neutron capture cross-section, releases Auger electrons, and gamma rays, which can cause localised cytotoxicity7. Moreover, multifunctional agents that may target tumours, deliver medications, and give real-time imaging are being created using gadolinium-based nanoparticles8. Gadolinium’s use in targeted therapy, imaging, and drug delivery is expanding beyond conventional limits, transforming personalised, and precision medicine as research advances in gadolinium-based chemicals9. Table 1 highlights the Gadolinium Clinical implications. This review aims to explore the emerging innovations, and biomedical applications of gadolinium-based compounds, focusing on their roles in diagnostic imaging-particularly magnetic resonance imaging (MRI)-and therapeutic interventions. It highlights recent advancements, discusses safety considerations, and evaluates the potential of gadolinium agents in next-generation theranostics.
Gadolinium-Based Contrast Agents (GBCAs)
Classification: Gadolinium-Based Contrast Agents (GBCAs) are used in MRI to improve diagnostic accuracy by shortening the T1 relaxation time of water protons. This allows radiologists to distinguish between normal, and pathological tissues, enabling the detection of tumours, inflammation, vascular abnormalities, and structural anomalies. Gadolinium, a rare-earth element with seven unpaired electrons, is safe for intravenous administration when chelated with stable organic ligands. The GBCAs are useful in neuroimaging, musculoskeletal studies, cardiovascular evaluations, oncology , and oncology for soft tissue contrast. However, safety concerns persist, especially in patients with renal impairment. The current review focuses on developing next-generation GBCAs with improved safety profiles, relaxivity, and targeted delivery capabilities10. Despite their effectiveness, the long-term safety has been reevaluated due to worries about gadolinium retention in the brain, and other tissues. The GBCAs have been linked to nephrogenic systemic fibrosis (NSF)11, an uncommon but crippling disease in people with severe renal failure. This concern has led to the issuance of patient screening guidelines, and the preference for macrocyclic medicines by regulatory organisations. One recent advancement is the creation of high-relaxivity drugs, like gadopiclenol, which enable lower dosages without sacrificing image quality. With possible uses in targeted imaging, and medication delivery, the integration of GBCAs into theranostic platforms, and nanoparticle-based delivery systems is also being researched. The safety profile, pharmacokinetics, and novel formulations of GBCAs are still being researched, and developed as MRI continues to change. Table 2 summarizes the pharmacokinetics, safety profiles, and diverse clinical applications of Gadolinium-Based Contrast Agents (GBCAs), highlighting their use in enhanced imaging12.
GBCAs are classified according to their ionic charge, and chemical structure: Macrocyclic versus Linear. While macrocyclic agents have a cage-like structure that firmly binds the gadolinium ion, and lowers the possibility of its release into the body, linear agents have an open-chain structure23,24. Ionic vs. Non-Ionic: While non-ionic agents are not charged, ionic agents are. Their osmolality, and risk of negative reactions are impacted by this difference25,26.
| Table 1: | Feature, and description of Gadolinium | |||
| Feature | Description |
| Central focus | Gadolinium as the core ion for imaging and therapy |
| Branching | Four major innovation areas: Contrast agents, Theranostics, Targeting, Safety |
| Theranostics | Emphasis on multifunctional platforms (e.g., Gd-based liposomes or dendrimers) |
| Targeted delivery | Includes bioconjugates for precision therapy (e.g., antibodies, sugars) |
| Safety emphasis | Deposition issues → solution strategies (macrocyclic ligands, alternative metals) |
| Clinical applications | Highlight MRI expansion into neurology, cancer, heart and multimodal fields |
| Table 2 | Pharmacokinetics, safety, and clinical applications of Gadolinium-Based Contrast Agents (GBCAs) | |||
| S. No. | Structure type | Pharmacokinetics and safety |
Clinical applications | Advances in GBCAs/ Nano-technology |
Reference |
| 1 | Linear non-ionic | Moderate stability, risk of Gd release |
Brain, liver imaging | - | Iyad et al.13 |
| 2 | Macrocyclic ionic | High stability, minimal NSF risk |
Neurology, | - | Ataur Rahman et al.14 |
| 3 | Macrocyclic non-ionic | Excellent safety profile | Whole-body MRI | - | Dufort et al.15 |
| 4 | Linear ionic | Less stable, potential Gd retention |
Angiography | - | Edwards et al.16 |
| 5 | Macrocyclic | Favorable PK, reduced brain deposition |
Oncologic imaging | Theranostics emerging | Clough et al.17 |
| 6 | Linear | Higher Gd dissociation rate |
Tumor perfusion | - | Wermuth and Jimenez18 |
| 7 | Macrocyclic | Minimal gadolinium deposition |
MS diagnosis | - | Loevner et al.19 |
| 8 | Macrocyclic | No confirmed NSF cases |
Liver lesion detection | - | Kuhl et al.20 |
| 9 | Linear | Reported brain Gd deposits |
Brain tumor assessment | - | Alsogati et al.21 |
| 10 | Macrocyclic | Excellent chelate stability |
Pediatric neuroimaging | - | Vymazal and Rulseh22 |
Mechanism of action: To increase the signal intensity on T1-weighted MRI images, GBCAs reduce the T1 relaxation time of adjacent hydrogen protons in water molecules. This improvement makes it possible to see blood vessels, tumours, and inflammatory areas more clearly27.
Mechanism of action: Gadolinium-Based Contrast Agents (GBCAs): By taking advantage of the paramagnetic characteristics of gadolinium ions (Gd3+)28,29, Gadolinium-Based Contrast Agents (GBCAs) improve picture contrast in magnetic resonance imaging (MRI). One of the most potent paramagnetic materials is gadolinium, which has seven unpaired electrons in its 4f orbital. This characteristic is essential to its MRI contrast-enhancing method of action30,31.
Chelation of gadolinium: Because free gadolinium ions disrupt cellular processes that depend on calcium, they are extremely hazardous. Gd3+ is firmly bound with a chelating ligand to reduce toxicity by blocking its contact with bodily tissues. Stability, and safety are impacted by the chelate’s ionic or non-ionic, linear or macrocyclic nature32,33.
Relaxivity and MRI signal enhancement: T1 relaxation time shortening is the main way that GBCAs function. The time it takes for protons in tissues-mostly water-to realign with the magnetic field following excitation by a radiofrequency pulse is known as T1 relaxation in MRI34,35. By boosting the longitudinal relaxation rate (1/T1) through dipole-dipole interactions with neighbouring water protons, gadolinium speeds up this realignment. Consequently, gadolinium-containing tissues show up as brighter on T1-weighted imaging36,37.
Water exchange and inner sphere mechanism: Inner sphere relaxivity, where water molecules are directly coupled to the Gd3+ ion, determines how effective a GBCA is. Second sphere, and outer sphere relaxivity: the presence of magnetic effects in the vicinity of water molecules that are not directly linked to Gd3+. Over time, Gd3+ can improve the relaxation of many protons because water molecules exchange in, and out of the coordination sphere at a rate of about 10-8 to 10-9 sec38.
Biodistribution and clearance: The majority of GBCAs are extracellular fluid agents that are found in the interstitial, and intravascular spaces39. Both the blood-brain, and blood-testis barriers remain intact. In people with normal renal function, GBCAs have a 1.5 hrs plasma half-life, and are mainly eliminated unaltered by the kidneys40,41.
Clinical applications of Gadolinium-Based Contrast Agents (GBCAs): Diagnostic imaging has been transformed by GBCAs, especially in neurology, identifying vascular anomalies, multiple sclerosis lesions, and brain malignancies42,43:
| • | Oncology: Recognising, and describing malignancies, evaluating the effectiveness of treatment, and spotting metastases44,45 | |
| • | Cardiology: Assessing the viability, and perfusion of the heart46 | |
| • | Musculoskeletal imaging: Evaluating infections, soft tissue malignancies, and joint diseases47 |
The particular clinical situation, patient characteristics, and the intended imaging results all influence the choice of GBCA. Since they allow for accurate anatomical, and functional imaging in a variety of medical specialties, Gadolinium-Based Contrast Agents or GBCAs, have become essential in clinical MRI practice48. In multiple sclerosis, contrast enhancement shows tumour margins, identifies recurrence, and tracks demyelinating plaques. Because of their strong relaxivity, and stable macrocyclic architectures, GBCAs like gadobutrol, and gadoterate have demonstrated great dependability49. GBCAs have a major positive impact on cardiovascular imaging, particularly in myocardial tissue characterisation, and MR angiography (MRA)50. Gadolinium-enhanced MRI helps in tumour staging, treatment planning, and assessing therapy response in oncology51. Hepatocyte uptake is provided by liver-specific GBCAs such as gadoxetate disodium (Eovist), which enhances the detection of Hepatocellular Carcinoma (HCC)52. Dynamic Contrast-Enhanced MRI (DCE-MRI) enhances lesion detection in breast cancer, particularly in high-risk groups or in dense breast tissue53. Applications in the musculoskeletal system include the assessment of bone marrow disease, soft tissue cancers, synovitis, and joint inflammation. Gadolinium-enhanced MRIs can identify postoperative problems in orthopaedic patients or distinguish between active inflammation, and chronic illness in rheumatoid arthritis54. Careful GBCA selection is essential despite their wide range of applications, especially for patients who need serial imaging or have renal impairment. Because of their better safety profiles, macrocyclic agents are typically chosen55. Figure 1 highlights the Gadolinium applications as a contrast agent in MRI to enhance the image quality, and visualize soft tissues, and Toxicity Concerns of
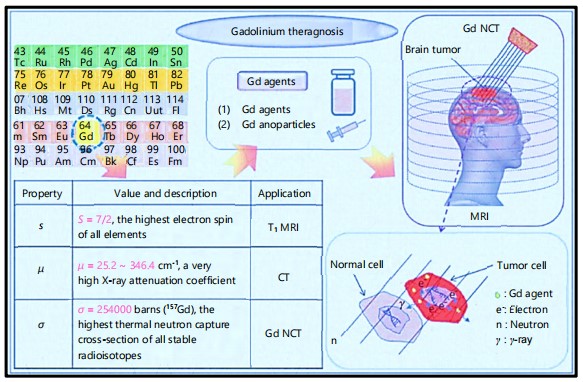
|
Gadolinium-Based Contrast Agents (GBCAs) Because of their exceptional capacity to improve image contrast, gadolinium-based contrast agents or GBCAs, have been employed extensively in MRI. GBCAs are risky, even though they are normally safe. Particularly with repeated use or in susceptible groups, worries concerning gadolinium retention, nephrogenic systemic fibrosis (NSF), and hypersensitivity reactions have gained more attention56.
Retention of gadolinium: Even in patients with normal renal function, recent research has demonstrated that trace levels of gadolinium can remain in the brain, bones, skin, and other organs for a considerable amount of time following GBCA delivery. By forming cage-like complexes that more firmly bind gadolinium, macrocyclic agents such as gadobutrol, gadoterate, and gadoteridol decrease the release, and retention of free Gd3+. Although gadolinium is present in tissues, there is currently no conclusive evidence linking retention to clinical damage in persons with normal renal function. However, care should be taken, particularly in groups that need MRI scans frequently57. Older linear nonionic drugs, such as gadodiamide, have a much higher risk of NSF. The use of these high-risk agents has been limited since 2007. When taken at the lowest effective dose, newer macrocyclic GBCAs are thought to be safe for patients with renal impairment, and exhibit a small incidence of NSF58,59.
Hypersensitivity and allergic reactions: Hypersensitivity reactions to GBCAs can range from minor rashes to severe anaphylaxis, albeit they are uncommon (affecting approximately 0.1 to 0.1% of individuals). With linear medicines, reactions occur more frequently, and are typically immediate (less than an hour after injection)59,60.
Paediatric and pregnancy considerations: Children’s lower glomerular filtration rates, and undeveloped renal function may cause gadolinium retention to last longer. Although there is little information on GBCAs’ teratogenicity, they pass through the placenta, and reach the foetal blood. GBCAs fall under pregnancy category C, and ought to be taken sparingly60,61.
Recent advances: Gadopiclenol, a next-generation, macrocyclic, non-ionic gadolinium-based contrast agent (GBCA), has been developed to offer superior imaging performance, and improved safety. Approved for clinical use in the US, and EU, gadopiclenol possesses high relaxivity, allowing it to produce stronger MRI signal enhancement at lower doses. Its macrocyclic structure reduces the risk of gadolinium ion release, which can cause adverse effects like nephrogenic systemic fibrosis. Gadopiclenol’s non-ionic nature also improves biocompatibility, and reduces allergic or hypersensitivity reactions. This makes it suitable for various clinical applications, including paediatric, and high-risk populations. Gadopiclenol sets a new benchmark in contrast agent development62.
CONCLUSION
Gadolinium’s unique paramagnetic, and luminescent properties make it invaluable in MRI diagnostics, and emerging cancer therapies. However, its clinical use is limited by toxicity concerns, especially in vulnerable patients. Recent innovations-such as gadolinium-loaded nanoparticles, smart ligands, and macromolecular carriers-aim to enhance safety, precision, and therapeutic utility. These advancements hold promise for safer, more effective imaging, and treatment strategies, ultimately improving diagnostic accuracy, and patient outcomes.
SIGNIFICANCE STATEMENT
The review article highlights the development of novel gadolinium-based nanoparticles, and complexes to improve the safety, and efficacy of gadolinium-based contrast agents in MRI. The gadolinium exhibits enhanced relaxivity, targeted delivery, and reduced toxicity, potentially improving MRI diagnostic accuracy, and enabling targeted therapy, especially for cancer treatment. This could lead to improved patient outcomes, and treatment options.
ACKNOWLEDGMENT
Authors are thankful to the S.M.B.T. College of Pharmacy, Maharashtra, India.
REFERENCES
- Starekova, J., A. Pirasteh and S.B. Reeder, 2024. Update on gadolinium-based contrast agent safety, from the AJR special series on contrast media. Am. J. Roentgenol., 223.
- Pan, D., A.H. Schmieder, S.A. Wickline and G.M. Lanza, 2011. Manganese-based MRI contrast agents: Past, present, and future. Tetrahedron, 67: 8431-8444.
- Dumazert, J., R. Coulon, Q. Lecomte, G.H.V. Bertrand and M. Hamel, 2018. Gadolinium for neutron detection in current nuclear instrumentation research: A review. Nucl. Instrum. Methods Phys. Res. Sect. A: Accel. Spectrometers Detectors Associated Equip., 882: 53-68.
- Song, Y., J. Zou, E.A. Castellanos, N. Matsuura and J.A. Ronald et al., 2024. Theranostics-A sure cure for cancer after 100 years? Theranostics, 14: 2464-2488.
- Robertson, A.G. and L.M. Rendina, 2021. Gadolinium theranostics for the diagnosis and treatment of cancer. Chem. Soc. Rev., 50: 4231-4244.
- Chargari, C., P. Maury, M. Texier, C. Genestie and P. Morice et al., 2024. Theragnostic gadolinium-based nanoparticles safely augment x-ray radiation effects in patients with cervical cancer. ACS Nano, 18: 16516-16529.
- Foshag, K., A.J. Tsiouris, M. Prince and M. Reichman, 2025. A review of gadolinium-based contrast agents in the setting of repeated MRI for high risk breast cancer screening. Clin. Imaging, 120.
- Oluwasola, I.E., A.L. Ahmad, N.F. Shoparwe and S. Ismail, 2022. Gadolinium based contrast agents (GBCAs): Uniqueness, aquatic toxicity concerns, and prospective remediation. J. Contam. Hydrol., 250.
- Li, H., Y. Zeng, H. Zhang, Z. Gu, Q. Gong and K. Luo, 2021. Functional gadolinium-based nanoscale systems for cancer theranostics. J. Controlled Release, 329: 482-512.
- Ho, S.L., H. Yue, T. Tegafaw, M.Y. Ahmad and S. Liu et al., 2022. Gadolinium neutron capture therapy (GdNCT) agents from molecular to nano: Current status and perspectives. ACS Omega, 7: 2533-2553.
- Kanygin, V., A. Zaboronok, A. Kichigin, E. Petrova and T. Guselnikova et al., 2023. Gadolinium neutron capture therapy for cats and dogs with spontaneous tumors using Gd-DTPA. Vet. Sci., 10.
- Matyskin, A.V., S.B. Angermeier, S.S. Drera, M.C. Prible, J.A. Geuther and M.D. Heibel, 2024. Actinium-225 photonuclear production in nuclear reactors using a mixed radium-226 and gadolinium-157 target. Nucl. Med. Biol., 136-137.
- Iyad, N., M.S. Ahmad, S.G. Alkhatib and M. Hjouj, 2023. Gadolinium contrast agents-challenges and opportunities of a multidisciplinary approach: Literature review. Eur. J. Radiol. Open, 11.
- Ataur Rahman, M., M. Jalouli, M.K. Yadab and M. Al-Zharani, 2025. Progress in drug delivery systems based on nanoparticles for improved glioblastoma therapy: Addressing challenges and investigating opportunities. Cancers, 17.
- Dufort, S., A. Bianchi, M. Henry, F. Lux and G. le Duc et al., 2015. Nebulized gadolinium-based nanoparticles: A theranostic approach for lung tumor imaging and radiosensitization. Small, 11: 215-221.
- Edwards, B.J., A.E. Laumann, B. Nardone, F.H. Miller and J. Restaino et al., 2014. Advancing pharmacovigilance through academic-legal collaboration: The case of gadolinium-based contrast agents and nephrogenic systemic fibrosis-A research on adverse drug events and reports (RADAR) report. Br. J. Radiol., 87.
- Clough, T.J., L. Jiang, K.L. Wong and N.J. Long, 2019. Ligand design strategies to increase stability of gadolinium-based magnetic resonance imaging contrast agents. Nat. Commun., 10.
- Wermuth, P.J. and S.A. Jimenez, 2011. Nephrogenic Systemic Fibrosis. In: Scleroderma: From Pathogenesis to Comprehensive Management, Varga, J., C.P. Denton and F.M. Wigley (Eds.), Springer, Boston, Massachusetts, ISBN: 978-1-4419-5774-0, pp: 137-159.
- Loevner, L.A., B. Kolumban, G. Hutóczki, K. Dziadziuszko, D. Bereczki, A. Bago and A. Pichiecchio, 2023. Efficacy and safety of gadopiclenol for contrast-enhanced MRI of the central nervous system. Invest. Radiol., 58: 307-313.
- Kuhl, C., T. Csőszi, W. Piskorski, T. Miszalski, J.M. Lee and P.M. Otto, 2023. Efficacy and safety of half-dose gadopiclenol versus full-dose gadobutrol for contrast-enhanced body MRI. Radiology, 308.
- Alsogati, E., H. Ghandourah and A. Bakhsh, 2023. Review of the efficacy and safety of gadopiclenol: A newly emerging gadolinium-based contrast agent. Cureus, 15.
- Vymazal, J. and A.M. Rulseh, 2024. MRI contrast agents and retention in the brain: Review of contemporary knowledge and recommendations to the future. Insights Imaging, 15.
- Choi, J.W. and W.J. Moon, 2019. Gadolinium deposition in the brain: Current updates. Korean J. Radiol., 20: 134-147.
- Bendszus, M., A. Laghi, J. Munuera, L.N. Tanenbaum, B. Taouli and H.C. Thoeny, 2024. MRI gadolinium-based contrast media: Meeting radiological, clinical, and environmental needs. Magn. Reson. Imaging, 60: 1774-1785.
- Rogosnitzky, M. and S. Branch, 2016. Gadolinium-based contrast agent toxicity: A review of known and proposed mechanisms. BioMetals, 29: 365-376.
- Frenzel, T., T. Wels, H. Pietsch, L. Schöckel, P. Seidensticker and J. Endrikat, 2025. Recent developments and future perspectives in magnetic resonance imaging and computed tomography contrast media. Invest. Radiol.
- Wahsner, J., E.M. Gale, A. Rodríguez-Rodríguez and P. Caravan, 2019. Chemistry of MRI contrast agents: Current challenges and new frontiers. Chem. Rev., 119: 957-1057.
- Guo, B.J., Z.L. Yang and L.J. Zhang, 2018. Gadolinium deposition in brain: Current scientific evidence and future perspectives. Front. Mol. Neurosci., 11.
- Lauenstein, T., F. Ramirez-Garrido, Y.H. Kim, S.E. Rha and J. Ricke et al., 2015. Nephrogenic systemic fibrosis risk after liver magnetic resonance imaging with gadoxetate disodium in patients with moderate to severe renal impairment: Results of a prospective, open-label, multicenter study. Invest. Radiol., 50: 416-422.
- Malayeri, A.A., K.M. Brooks, L.H. Bryant, R. Evers, P. Kumar, D.S. Reich and D.A. Bluemke, 2016. National Institutes of Health perspective on reports of gadolinium deposition in the brain. J. Am. Coll. Radiol., 13: 237-241.
- Baranger, J., O. Villemain, M. Wagner, M. Vargas-Gutierrez and M. Seed et al., 2021. Brain perfusion imaging in neonates. NeuroImage: Clin., 31.
- Fraum, T.J., D.R. Ludwig, M.R. Bashir and K.J. Fowler, 2017. Gadolinium-based contrast agents: A comprehensive risk assessment. Magn. Reson. Imaging, 46: 338-353.
- Wei, Y., X. Jiang, M. Hibberd, A. Sampedro and J. Rautenbach, 2025. Estimating the rate of acute adverse reactions to non-ionic low-osmolar contrast media: A systematic review and meta-analysis. Eur. Radio.
- Iacobellis, F., M. di Serafino, C. Russo, R. Ronza and M. Caruso et al., 2024. Safe and informed use of gadolinium-based contrast agent in body magnetic resonance imaging: Where we were and where we are. J. Clin. Med., 13.
- Do, C., J. deAguero, A. Brearley, X. Trejo, T. Howard, G.P. Escobar and B. Wagner, 2020. Gadolinium-based contrast agent use, their safety, and practice evolution. Kidney360, 1: 561-568.
- Coimbra, S., S. Rocha, N.R. Sousa, C. Catarino and L. Belo et al., 2024. Toxicity mechanisms of gadolinium and gadolinium-based contrast agents-A review. Int. J. Mol. Sci., 25.
- Scarciglia, A., C. Papi, C. Romiti, A. Leone, E. di Gregorio and G. Ferrauto, 2025. Gadolinium-based contrast agents (GBCAs) for MRI: A benefit-risk balance analysis from a chemical, biomedical, and environmental point of view. Global Challenges, 9.
- Baykara, M., M. Ozcan, M. Bilgen and H. Kelestimur, 2019. Effects of gadolinium and gadolinium chelates on intracellular calcium signaling in sensory neurons. Neurosci. Lett., 707.
- Gaeta, M., K. Galletta, M. Cavallaro, E. Mormina and M.T. Cannizzaro et al., 2024. T1 relaxation: Chemo-physical fundamentals of magnetic resonance imaging and clinical applications. Insights Imaging, 15.
- Kanal, E., J.H. Maki, P. Schramm and L. Marti-Bonmati, 2025. Evolving characteristics of gadolinium-based contrast agents for MR imaging: A systematic review of the importance of relaxivity. Magn. Reson. Imaging, 61: 52-69.
- Maimouni, I., C. Henoumont, M.C. de Goltstein, J.F. Mayer and A. Dehimi et al., 2025. Gadopiclenol: A q = 2 gadolinium-based MRI contrast agent combining high stability and efficacy. Invest. Radiol., 60: 234-243.
- Davies, J., P. Siebenhandl-Wolff, F. Tranquart, P. Jones and P. Evans, 2022. Gadolinium: Pharmacokinetics and toxicity in humans and laboratory animals following contrast agent administration. Arch. Toxicol., 96: 403-429.
- Davies, J., P. Siebenhandl-Wolff, F. Tranquart, P. Jones and P. Evans, 2022. Correction to: Gadolinium: Pharmacokinetics and toxicity in humans and laboratory animals following contrast agent administration. Arch. Toxicol., 96: 1491-1491.
- Rovira, À., F.M. Doniselli, C. Auger, L. Haider and J. Hodel et al., 2024. Use of gadolinium-based contrast agents in multiple sclerosis: A review by the ESMRMB-GREC and ESNR Multiple Sclerosis Working Group. Eur. Radiol., 34: 1726-1735.
- Quattrocchi, C.C., M. Parillo, F. Spani, D. Landi and G. Cola et al., 2023. Skin thickening of the scalp and high signal intensity of dentate nucleus in multiple sclerosis: Association with linear versus macrocyclic gadolinium-based contrast agents administration. Invest. Radiol., 58: 223-230.
- Al-Sabeq, B., F. Nabi and D.J. Shah, 2019. Assessment of myocardial viability by cardiac MRI. Curr. Opin. Cardiol., 34: 502-509.
- Elawad, A., A. Shah, M. Davies and R. Botchu, 2021. The use of gadolinium in musculoskeletal MRI-Time to rethink? Indian J. Radiol. Imaging, 31: 635-643.
- Smith, T.E., A. Steven and B.A. Bagert, 2019. Gadolinium deposition in neurology clinical practice. Ochsner J., 19: 17-25.
- Meier, C., M. Eisenblätter and S. Gielen, 2024. Myocardial late gadolinium enhancement (LGE) in cardiac magnetic resonance imaging (CMR)-An important risk marker for cardiac disease. J. Cardiovasc. Dev. Dis., 11.
- Rong, D., B. He, W. Tang, S. Xie and S. Kuang et al., 2022. Comparison of gadobenate-enhanced MRI and gadoxetate-enhanced MRI for hepatocellular carcinoma detection using LI-RADS version 2018: A prospective intraindividual randomized study. Am. J. Roentgenol., 218: 687-698.
- Youssef, M.A., H.M.S. Elahwal, M.M. Alwageeh and S.E. Attya, 2018. Role of MRI in differentiating benign from malignant breast lesions using dynamic contrast enhanced MRI and diffusion weighted MRI. Alexandria J. Med., 54: 1-9.
- McDonald, R.J., D. Levine, J. Weinreb, E. Kanal and M.S. Davenport et al., 2018. Gadolinium retention: A research roadmap from the 2018 NIH/ACR/RSNA workshop on gadolinium chelates. Radiology, 289: 517-534.
- Dekker, H.M., G.J. Stroomberg, A.J. van der Molen and M. Prokop, 2024. Review of strategies to reduce the contamination of the water environment by gadolinium-based contrast agents. Insights Imaging, 15.
- Si, G., Y. Du, P. Tang, G. Ma and Z. Jia et al., 2024. Unveiling the next generation of MRI contrast agents: Current insights and perspectives on ferumoxytol-enhanced MRI. Natl. Sci. Rev., 11.
- Bonafè, R., A. Coppo, R. Queliti, S. Bussi, F. Maisano, M.A. Kirchin and F. Tedoldi, 2023. Gadolinium retention in a rat model of subtotal renal failure: Are there differences among macrocyclic GBCAs? Eur. Radio. Exp., 7.
- Bussi, S., A. Coppo, C. Botteron, V. Fraimbault and A. Fanizzi et al., 2018. Differences in gadolinium retention after repeated injections of macrocyclic MR contrast agents to rats. Magn. Reson. Imaging, 47: 746-752.
- Schieda, N., J.I. Blaichman, A.F. Costa, R. Glikstein and C. Hurrell et al., 2018. Gadolinium-based contrast agents in kidney disease: A comprehensive review and clinical practice guideline issued by the Canadian Association of Radiologists. Can. J. Kidney Health Dis., 5.
- Bara, M.T.G., A. Gallardo-Higueras, E.M. Moreno, E. Laffond and F.J.M. Bellido et al., 2022. Hypersensitivity to gadolinium-based contrast media. Front. Allergy, 3.
- Fok, J.S. and W.B. Smith, 2017. Hypersensitivity reactions to gadolinium-based contrast agents. Curr. Opin. Allergy Clin. Immunol., 17: 241-246.
- Stanescu, A.L., D.W. Shaw, N. Murata, K. Murata, J.C. Rutledge, E. Maloney and K.R. Maravilla, 2020. Brain tissue gadolinium retention in pediatric patients after contrast-enhanced magnetic resonance exams: Pathological confirmation. Pediatr. Radiol., 50: 388-396.
- Alghamdi, S.A., 2023. Gadolinium-based contrast agents in pregnant women: A literature review of MRI safety. Cureus, 15.
- Hao, J., C. Pitrou and P. Bourrinet, 2024. A comprehensive overview of the efficacy and safety of gadopiclenol: A new contrast agent for MRI of the CNS and body. Invest. Radiol., 59: 124-130.
How to Cite this paper?
APA-7 Style
Kumbhare,
M.R., Narkhede,
H.I., Dighe,
P.R., Chandak,
S. (2025). Emerging Innovations in Gadolinium-Based Imaging and Therapy. Trends in Pharmacology and Toxicology, 1(1), 40-48. https://doi.org/10.21124/tpt.2025.40.48
ACS Style
Kumbhare,
M.R.; Narkhede,
H.I.; Dighe,
P.R.; Chandak,
S. Emerging Innovations in Gadolinium-Based Imaging and Therapy. Trends Pharm. Toxicol. 2025, 1, 40-48. https://doi.org/10.21124/tpt.2025.40.48
AMA Style
Kumbhare
MR, Narkhede
HI, Dighe
PR, Chandak
S. Emerging Innovations in Gadolinium-Based Imaging and Therapy. Trends in Pharmacology and Toxicology. 2025; 1(1): 40-48. https://doi.org/10.21124/tpt.2025.40.48
Chicago/Turabian Style
Kumbhare, Manoj, R., Harsha I. Narkhede, Pravin R. Dighe, and Siddhi Chandak.
2025. "Emerging Innovations in Gadolinium-Based Imaging and Therapy" Trends in Pharmacology and Toxicology 1, no. 1: 40-48. https://doi.org/10.21124/tpt.2025.40.48

This work is licensed under a Creative Commons Attribution 4.0 International License.




