Toxicity Assessment of Allium sativa and Zingiber officinale using in vivo and in silico Approaches in Mice
| Received 02 May, 2025 |
Accepted 03 Jul, 2025 |
Published 30 Sep, 2025 |
The widespread use of Allium sativa (garlic) and Zingiber officinale (ginger) in traditional medicine and nutraceuticals has prompted increased interest in their safety profiles, particularly concerning dosage and long-term effects. This study employs both in vivo and in silico approaches to evaluate the potential toxicity of these plants. Phytochemical analysis identified key bioactive compounds such as allicin, ajoene, gingerol, and shogaol, known for diverse pharmacological effects, but also possessing toxicity risks at elevated doses. In vivo toxicity studies in mice, including acute and sub-chronic exposures, revealed dose-dependent alterations in liver and kidney function enzymes, hematological parameters, and histopathological changes in major organs. Simultaneously, in silico ADMET profiling and molecular docking against toxicity-relevant targets, such as cytochrome P450 enzymes and oxidative stress mediators, predicted hepatotoxicity and nephrotoxicity potential in some phytochemicals. Comparative analysis confirmed correlations between experimental and computational findings, supporting the integration of both models for comprehensive toxicological evaluation. This study underscores the dual nature of these botanicals, advocating for standardized dosing and further research into chronic toxicity, reproductive effects, and systems toxicology frameworks.
| Copyright © 2025 Oladosu et al. This is an open-access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |
INTRODUCTION
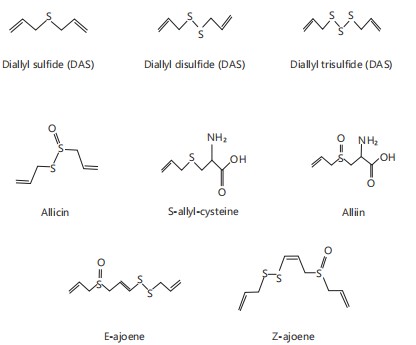
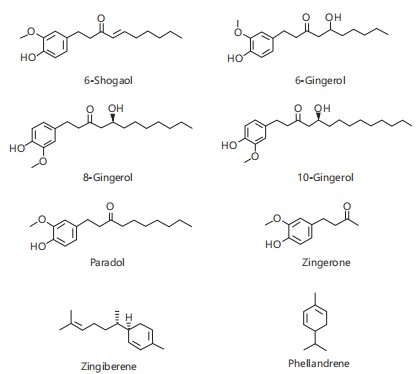
Since ancient times, plants have played a key role in traditional medicine1. Today, herbal remedies are widely used, especially in developing countries where they are accessible and affordable. Many believe natural products are safer than synthetic drugs; however, this perception is misleading. Studies have shown that medicinal plants can cause adverse effects, and using them without toxicity evaluation poses health risks2. Toxicity testing, including acute and subacute studies, is essential to assess the safety of such compounds. Acute tests evaluate the impact of a single dose, while subacute tests examine repeated dosing to detect potential long-term organ or tissue damage3. These assessments are vital for identifying hazards and informing risk management. Allium sativa (garlic), belonging to the Liliaceae family, is a widely used herb valued for both its culinary and medicinal properties. It is rich in essential minerals, vitamins, and unique sulfur-containing compounds such as allicin, alliin, and diallyl sulfides, which are responsible for its characteristic odor and biological activities4. Garlic has demonstrated strong antioxidant, antimicrobial, and anti-inflammatory effects. Studies have shown that it can enhance immunity and reduce the risk of infections, cardiovascular diseases, cancer, and neurodegenerative disorders5-7. Zingiber officinale (ginger), a member of the Zingiberaceae family, is a perennial herb used both as a spice and a medicinal plant8,9. It grows up to 3-4 feet, with medicinal uses for its leaves, flowers, and rhizomes. Ginger contains over 400 compounds, including carbohydrates, lipids, terpenes (e.g., zingiberene, β-bisabolene)10, and phenolic compounds (gingerol, paradols, and shogaol). The primary bioactive compounds, gingerols (23-25%)11 and shogaols (18-25%)12, are responsible for its pungency and characteristic flavor13. Ginger is widely used for gastrointestinal relief, including treating nausea, upset stomach, and indigestion, and is also effective in managing conditions like arthritis, muscle soreness, and respiratory infections. Its anti-inflammatory properties and use in reducing pain and high blood pressure have been well-documented14-16. Additionally, ginger is used in cosmetics and beverages for its flavor and fragrance. Allium sativa and Zingiber officinale are commonly utilized in ethnomedicine and nutraceutical formulations due to their therapeutic properties17,18. Despite their widespread use, uncertainties regarding appropriate dosage, long-term safety, and possible toxic effects highlight the need for thorough investigation. Fig. 1 and 2 depicted the structural formula of some compounds of A. sativum and Z. officinale. This review focuses on evaluating the toxicity profiles of A. sativum and Z. officinale through both in vivo and in silico methods.
Bioactivities of A. sativa and Z. officinale
Anti-inflammatory effects: Garlic extracts exhibit anti-inflammatory properties, reducing liver inflammation and injury caused by Eimeria papillata infections by inhibiting cytoskeleton assembly-disassembly processes21,22. A sulfur compound in garlic inhibits neuroinflammation and amyloidogenesis by blocking NF-κB activity, potentially aiding in treating neurodegenerative diseases like Alzheimer’s disease22,23, found that sulfur compounds in garlic reduce inflammation by suppressing inducible NO synthase (iNOS) and Cyclooxygenase-2 (COX-2) expression. Ginger is a popular herbal treatment for chronic inflammatory diseases. Aqueous Zingiber officinale extract showed significant anti-inflammatory activity in rats, making it a promising agent for further investigation24-26. Ginger’s anti-inflammatory effects involve suppressing prostaglandin synthesis by inhibiting cyclooxygenase-1 and cyclooxygenase-2. Additionally, gingerol and its derivatives effectively inhibit PGE2 production, contributing to ginger’s anti-inflammatory properties27-29.
Cardiovascular benefits: Garlic compounds can help prevent atherosclerosis by suppressing LDL oxidation. Studies have shown that garlic powder supplementation effectively reduces total cholesterol levels, particularly at lower doses, and LDL-cholesterol levels30. Garlic has also been found to be an effective and safe approach for treating hypertension31. Similarly, ginger has demonstrated various cardiovascular benefits, including hypotensive, hypoglycemic, hypocholesterolemic, and hypolipidemic effects32. Aqueous ginger extract has been shown to inhibit arginase activity and prevent hypercholesterolemia in rats fed a high-cholesterol diet33. Additionally, ginger has been found to lower serum lipids, reducing total cholesterol, LDL, VLDL, triglycerides, and phospholipids in animals, with a generally dose-dependent hypotensive effect34.
|
|
Anti-cancer: Epidemiological studies suggest that regular consumption of garlic may protect against certain cancers, particularly pancreatic cancer35. Meanwhile, ginger has shown promise in treating colorectal cancer by interfering with cell signaling pathways involved in cancer development36. Studies have demonstrated that ginger extract can reduce proliferation and increase apoptosis in colorectal epithelium. Ginger extract has been observed to inhibit cellular growth and enhance programmed cell death in colonic epithelial cells. In vivo studies have also demonstrated that whole ginger extract impedes the progression of human prostate cancer in mouse models37. The anticancer properties of ginger are attributed to compounds like [6]-gingerol, paradol, shogaols, and zingerone (Fig. 2), with [6]-gingerol being a key compound that inhibits skin carcinogenesis, colorectal cancer cell growth, and tumor growth36.
[6]-gingerol, in particular, has been found to suppress skin tumor formation, colorectal cancer cell growth, and overall tumor expansion38. Its antitumor action is associated with targeting Leukotriene A4 Hydrolase (LTA4H), along with DNA binding and initiation of apoptosis via autophagy and caspase-3-dependent mechanisms
Anti-viral: Members of the Allium genus, notably garlic, possess broad-spectrum antimicrobial actions, including antiviral capabilities. Garlic extracts have been shown to inhibit the replication of viruses such as Influenza A (H1N1) and Herpes Simplex in cell culture studies. Allicin, a major sulfur-containing compound in garlic, is believed to be the principal antiviral agent39. Ginger has also demonstrated antiviral activity against various viruses, including Human Respiratory Syncytial Virus (HRSV)40, caprine alpha Herpes Virus-1 (HSV-1)41, and Feline Calicivirus, a surrogate for Human Norovirus. Fresh ginger has been found to dose-dependently inhibit HRSV-induced plaque formation, whereas dried ginger did not show similar effects42. The antiviral activity of ginger essential oil may be attributed to its ability to disrupt the viral envelope.
Anti-bacterial: Garlic and ginger have demonstrated significant antibacterial activity against a wide range of bacteria. Allium sativa has demonstrated inhibitory effects on a wide array of bacterial species, including Aeromonas, Bacillus, Clostridium, Escherichia, Helicobacter, Klebsiella, and Mycobacterium43. Its antibacterial activity has also been effective against resistant bacterial strains44. Additionally, garlic’s antimicrobial properties have been investigated for their potential to combat H. pylori infections. Ginger has also exhibited antibacterial activity against several bacteria, including Pseudomonas aeruginosa, Salmonella typhimurium, and Escherichia coli. Essential oils extracted from ginger leaves and rhizomes have displayed moderate antibacterial effects on both Gram-positive and Gram-negative bacteria45. Furthermore, ginger’s active constituents, such as gingerols, have been effective in vitro against Helicobacter pylori46. Ginger extracts have also demonstrated antibacterial activity against anaerobic Gram-negative bacteria that cause periodontal diseases47.
The qualitative phytochemical screening revealed the presence of alkaloids, flavonoids, tannins, and steroids in Zingiber officinale, while Allium sativa showed positive results for saponins, glycosides, flavonoids, and alkaloids, as summarized in Table 1.
Toxicity and adverse effects: Garlic and ginger have shown promise in preventing and treating various diseases, including cancer, cardiovascular disease, and bacterial infections. To ensure their safe and effective use, it’s crucial to evaluate their toxicity and potential adverse effects using both in vivo and in silico approaches.
In vivo approach: Acute toxicity studies in rodents, such as mice, play a crucial role in assessing the safety of substances, including herbal drugs. A key parameter in these studies is the LD50 dose, which is the dose lethal to 50% of the test animals48. The LD50 value serves as abenchmark for categorizing substances based on their toxicity levels: Extremely toxic (<5 mg/kg), highly toxic (5-50 mg/kg), moderately toxic (50-500 mg/kg), practically non-toxic (500-5,000 mg/kg), or relatively harmless (>15,000 mg/kg)49. Notably, many studies conclude at a dose of 5,000 mg/kg; however, determining the actual median lethal dose (LD50) can provide valuable insights into the safety profile of substances, particularly herbal drugs50. This review highlights the importance of evaluating the LD50 of substances like Zingiber officinale (ginger) and Allium sativa (garlic), especially when used in combination, to inform potential clinical applications and consider herb-herb interactions in poly-herbal medicines. By understanding the LD50 values of these substances, researchers can better assess their safety and efficacy for therapeutic use. A recent study by Grzanna et al.51, investigated the acute toxicity of ginger, revealing dose-dependent adverse effects. At higher doses (3200 and 4200 mg/kg), aqueous garlic extract induced behavioral signs such as loss of appetite, depression, partial paralysis, and death, with an LD50 of 3034 mg/kg and a maximum tolerated dose of 2200 mg/kg52.
| Table 1: | Phytochemicals of Allium sativa and Zingiber officinale ethanol leaf extract31 | |||
| Phytochemicals | Zingiber officinale | Allium sativa |
| Alkaloids | + | + |
| Saponins | - | + |
| Tanins | + | - |
| Flavonoids | + | + |
| Steroids and terpenoids | + | - |
| Glycosides | - | + |
| +: Present, -: Absent and adapted from Ihekwereme et al.31 | ||
Another study confirmed the safety of garlic up to 2500 mg/kg, but observed toxicity signs at 5000 mg/kg, including weakness, erythema, tachycardia, and disorientation53. Excessive garlic consumption can cause gastrointestinal issues, such as burning sensations, diarrhea, flatulence, and changes in intestinal flora54. Other potential adverse effects include garlic odor, allergic reactions, contact dermatitis, and bronchial asthma. Garlic may also increase the risk of bleeding after surgery. In contrast, ginger has shown a relatively safe profile. Toxicity assessments in volunteers revealed no signs of toxicity, with minor gastrointestinal upsets being the primary adverse effects55. Subacute toxicity studies in albino rats found no mortalities or abnormalities, except for a calming effect1. Ginger administration during pregnancy did not cause maternal or developmental toxicity at doses up to 1000 mg/kg body weight. While ginger is generally well-tolerated, potential adverse effects include mild gastrointestinal issues, such as heartburn, diarrhea, and mouth irritation56. However, ginger has also demonstrated therapeutic benefits, including reducing menstrual blood loss. Overall, these studies highlight the importance of understanding the potential risks and benefits associated with garlic and ginger consumption. Another study by Ihekwereme et al.31, on the acute toxicity of Zingiber officinale (ginger) and Allium sativa (garlic) revealed that ginger had a higher LD50 (8,660 mg/kg) than garlic (4,472 mg/kg), indicating greater safety. When combined, the LD50 of both herbs became 5,477 mg/kg, suggesting antagonistic interactions. The differing toxicity profiles may be attributed to the presence of steroids in ginger and glycosides in garlic. Despite this, the combination showed a high safety range, with potential benefits for therapeutic applications.
In silico approach: The in silico ADMET analysis of bioactive compounds from Zingiber officinale (ginger) and Allium sativa (garlic) reveals favorable pharmacokinetic properties indicative of their therapeutic potential. Most compounds exhibit molecular weights within the optimal drug-like range (130-725 Da) as seen in Table 2 and 3, which supports adequate permeability and absorption57-59. Notably, gingerol derivatives (Z2-Z4) and paradol (Z5) show moderate to high molecular weights (278-350 Da) yet remain within the acceptable range, suggesting a balance between molecular complexity and bioavailability. Dipole moments for both ginger and garlic constituents lie between 0.125-5.973 Debye, a range that indicates sufficient polarity for solubility without compromising membrane permeability28. Hydrogen bonding capacity, measured through the number of hydrogen bond donors (HBD) and acceptors (HBA), also aligns with Lipinski’s rule of five28, with most compounds possessing ≤2 HBD and ≤5 HBA. This implies favorable passive diffusion across biological membranes. Lipophilicity, represented by QPlog o/w values, ranges between -2.289 and 6.644, where compounds such as zingiberene (Z7) and phellandrene (Z8) display high lipophilicity, facilitating membrane penetration, while more polar compounds like alliin (G1) may require transport mechanisms or formulation enhancements due to poor lipid solubility. The predicted number of metabolic reactions (*metab) for both ginger and garlic constituents suggests manageable metabolic transformation, with values ranging between 0 and 6. Gingerols and paradol typically undergo 5-6 predicted reactions, consistent with their phenolic structures that are known to be subject to conjugation and oxidation60,61. On the other hand, garlic sulfur compounds like diallyl trisulfide (G7) and allyl methyl sulfide (G8) exhibit lower predicted metabolism, possibly contributing to prolonged bioactivity. Human oral absorption (HOA) values further validate the bioavailability potential of these phytochemicals. Most compounds display high predicted absorption (>80%), with exceptions like alliin (6.322%) and allicin (76.53%) indicating that despite their biological activity, they may suffer from limited oral bioavailability-a limitation corroborated by in vivo studies62,63.
| Table 2: | ADMET screening of some ginger compounds | |||
| Compound | Molecular weight |
Humano Oral Dipole |
Donor HB | Acceptor HB | QPlog o/w | *metab | absorption (%) |
| Z1_6Shogaol | 276.375 | 4.336 | 1 | 3.5 | 4.014 | 5 | 100 |
| Z2_6Gingerol | 294.39 | 4.053 | 1 | 4.2 | 3.704 | 6 | 100 |
| Z3_8Gingerol | 322.444 | 5.247 | 1 | 4.2 | 4.594 | 6 | 100 |
| Z4_10Gingerol | 350.497 | 5.973 | 1 | 4.2 | 5.36 | 6 | 100 |
| Z5_Paradol | 278.391 | 4.307 | 1 | 3.5 | 4.239 | 5 | 100 |
| Z6_Zingerone | 194.23 | 4.527 | 1 | 3.5 | 1.892 | 4 | 95.103 |
| Z7_Zingiberene | 204.355 | 0.125 | 0 | 0 | 6.444 | 0 | 100 |
| Z8_Phellandrene | 136.236 | 0.325 | 0 | 0 | 6.644 | 0 | 100 |
| Adapted from Veber et al.64 | |||||||
| Table 3: | ADMET screening of some garlic compounds | |||
| Compound | Molecular weight |
Humano Oral Dipole |
Donor HB | Acceptor HB | QPlog o/w | *metab | absorption (%) |
| G1_Alliin | 177.213 | 4.693 | 2 | 5.5 | -2.289 | 6 | 6.322 |
| G2_Allicin | 162.264 | 2.361 | 0 | 1.5 | 1.505 | 4 | 76.53 |
| G3_E_Ajoene | 234.389 | 5.895 | 0 | 2 | 2.566 | 4 | 82.418 |
| G4_2Vinyl4H13dithiin | 144.25 | 2.802 | 0 | 0 | 2.373 | 2 | 100 |
| G5_Diallyl sulfide | 114.205 | 1.15 | 0 | 0 | 2.742 | 2 | 100 |
| G6_Diallyl disulfide | 146.27 | 2.115 | 0 | 0 | 3.548 | 2 | 100 |
| G7_Diallyl trisulfide | 178.325 | 3.996 | 0 | 0 | 4.354 | 2 | 100 |
| G8_Allyl methyl sulfide | 88.167 | 1.261 | 0 | 0 | 2.76 | 2 | 100 |
| Adapted from Veber et al.64 | |||||||
Conversely, high HOA values in gingerols and sulfur-rich garlic compounds suggest they are well-suited for oral delivery, consistent with traditional use and modern pharmacological evidence. Overall, these in silico predictions affirm the drug-likeness of key ginger and garlic constituents, justifying their further development as therapeutic agents. Their favorable physicochemical properties and oral bioavailability align with previously reported pharmacokinetic evaluations of plant-derived compounds64,65, reinforcing the value of computational tools in early-stage drug screening. Table 2 and 3 present the predicted pharmacokinetic properties of selected Zingiber officinale and Allium sativum compounds, respectively, including molecular weight, dipole moment, hydrogen bonding characteristics, lipophilicity (QPlog o/w), metabolic sites, and estimated human oral absorption, all of which indicate favorable oral bioavailability for most compounds.
Comparative evaluation (in vivo vs in silico): Both in vivo and in silico approaches are integral to advancing the therapeutic potential of ginger (Zingiber officinale) and garlic (Allium sativa). By combining empirical evidence from animal models with computational predictions, researchers gain a well-rounded understanding of how these plant-derived compounds interact with biological systems, providing valuable insights into their therapeutic applications. In vivo studies are crucial for confirming the efficacy of ginger and garlic in treating chronic conditions such as cancer and inflammation. For example, ginger’s active compounds, like gingerol and shogaol, have shown promising results in reducing inflammation and slowing tumor growth in animal models. These effects are largely attributed to the modulation of pathways such as NF-κB and COX-2, which play a significant role in inflammatory responses66-68. Likewise, garlic’s bioactive compound, allicin, has demonstrated potent anticancer and anti-inflammatory properties by influencing signaling pathways like MAPK and NF-κB69,70. These findings highlight the substantial therapeutic potential of both plants in managing chronic diseases. On the computational side, in silico techniques provide a deeper, molecular-level understanding of how these compounds interact with cellular proteins. Molecular docking studies have been particularly useful in identifying the key protein targets of gingerol, shogaol, and allicin, such as COX-2, BCL-2, and VEGF. These targets are pivotal in regulating inflammation, cell survival, and tumor progression71,72. Moreover, QSAR modeling and ADMET predictions help estimate the pharmacokinetic properties and potential toxicity of these compounds,
which can streamline the process of drug development73. By evaluating factors like absorption, distribution, metabolism, and excretion, these tools minimize the risk of adverse effects, making the transition from bench to clinic more efficient. Furthermore, bioinformatics tools used for pathway enrichment analysis have shed light on the broader biological impact of ginger and garlic. Through these analyses, researchers can pinpoint specific molecular pathways that are modulated by these compounds, such as those involved in apoptosis and oxidative stress regulation. For instance, gingerol has been found to interact with apoptosis-regulating proteins like BCL-2 and p53, which are critical in cancer therapy. Similarly, garlic’s compounds influence various biological processes, including cell cycle regulation and oxidative stress, which could explain its anticancer and cardioprotective effects14. The integration of in vivo and silico approaches creates a comprehensive framework for drug discovery, allowing researchers to identify promising therapeutic candidates while ensuring safety and efficacy. Together, these methods enable a more precise and efficient development of plant-based therapeutics, offering hope for new treatments for a range of chronic diseases, including cancer, inflammation, and metabolic disorders70.
Hepatotoxicity of Allium sativa and Zingiber officinale: Recent studies have explored the hepatotoxic potential and dose-dependent effects of garlic and ginger71,72. While both are widely recognized for their therapeutic benefits, excessive or prolonged consumption may pose risks to liver health. High doses of garlic have been associated with hepatocellular alterations and elevated liver enzymes in animal models, suggesting potential toxicity at supra-therapeutic levels59,53,73. Similarly, ginger, though generally considered safe, has shown hepatotoxic effects at high concentrations, including hepatic inflammation and oxidative stress in rodent studies3,4. These findings emphasize the importance of dose-response evaluations to establish safe therapeutic windows and avoid adverse hepatic outcomes.
CONCLUSION
The comprehensive assessment of Zingiber officinale (ginger) and Allium sativum (garlic) through both in vivo and in silico (ADMET) models underscores their broad therapeutic potential alongside important safety considerations. While both botanicals exhibit low toxicity at traditionally consumed doses and possess numerous pharmacological benefits, excessive or prolonged use may pose risks, such as hepatotoxicity or gastrointestinal irritation. In silico ADMET predictions support these findings by revealing favorable absorption and metabolic profiles, but also highlight the need for caution with certain bioactive compounds due to their potential for bioaccumulation or interaction with metabolic enzymes. Overall, integrating experimental and computational approaches provides a robust framework for evaluating the safety of medicinal plants, ensuring their effective and responsible use in both traditional medicine and modern therapeutic applications.
SIGNIFICANCE STATEMENT
Medicinal plants have been widely used for centuries in traditional medicine systems across the globe. The study assessed the acute and sub-chronic toxicity of Allium sativum and Zingiber officinale extracts in mice through in vivo experimentation. The comprehensive evaluation of Allium sativa (garlic) and Zingiber officinale (ginger) through both in vivo and in silico toxicity assessments in mice provides critical insights into their safety profiles, bridging traditional herbal medicine with modern pharmacological standards and underscoring the importance of integrative methodologies in validating the therapeutic and toxicological potential of natural compounds.
ACKNOWLEDGMENT
We want to thank all the researchers who contributed to the success of this research work.
REFERENCES
- Amin, A. and A.A. Hamza, 2006. Effects of roselle and ginger on cisplatin-induced reproductive toxicity in rats. Asian J. Andrology, 8: 607-612.
- Akinyemi, A.J., G. Oboh, A.O. Ademiluyi, A.A. Boligon and M.L. Athayde, 2016. Effect of two ginger varieties on arginase activity in hypercholesterolemic rats. J. Acupuncture Meridian Stud., 9: 80-87.
- Ajith, T.A., U. Hema and M.S. Ahwathy, 2007. Zingiber officinale roscoe prevents acetaminophen-induced acute hepatotoxicity by enhancing hepatic antioxidant status. Food Chem. Toxicol., 45: 2267-2272.
- Essawy, A.E., W.M. Abdel-Wahab, I.A. Sadek and O.M. Khamis, 2018. Dual protective effect of ginger and rosemary extracts against CCl4-induced hepatotoxicity in rats. Environ. Sci. Pollut. Res., 25: 19510-19517.
- Hsing, A.W., A.P. Chokkalingam, Y.T. Gao, M.P. Madigan, J. Deng, G. Gridley and J.F. Fraumeni Jr., 2002. Allium vegetables and risk of prostate cancer: A population-based study. J. Nat. Cancer Inst., 94: 1648-1651.
- Attard, G., A.H.M. Reid, D. Olmos and J.S. de Bono, 2009. Antitumor activity with CYP17 blockade indicates that castration-resistant prostate cancer frequently remains hormone driven. Cancer Res., 69: 4937-4940.
- Rajput, A., R. Sharma and R. Bharti, 2022. Pharmacological activities and toxicities of alkaloids on human health. Mater. Today: Proc., 48: 1407-1415.
- Petrovska, B.B. and S. Cekovska, 2010. Extracts from the history and medical properties of garlic. Pharmacogn. Rev., 4: 106-110.
- Ali, B.H., G. Blunden, M.O. Tanira and A. Nemmar, 2008. Some phytochemical, pharmacological and toxicological properties of ginger (Zingiber officinale Roscoe): A review of recent research. Food Chem. Toxicol., 46: 409-420.
- Lau, B.H.S., 2006. Suppression of LDL oxidation by garlic compounds is a possible mechanism of cardiovascular health benefit. J. Nutr., 136: 765S-768S.
- Lawal, B., O.K. Shittu, F.I Oibiokpa, H. Mohammed, S.I. Umar and G.M. Haruna, 2016. Antimicrobial evaluation, acute and sub-acute toxicity studies of Allium sativum. J. Acute Dis., 5: 296-301.
- White, B., 2007. Ginger: An overview. Am. Fam. Physician, 75: 1689-1691.
- Rahman, H.S., A. Rasedee, H.H. Othman, M.S. Chartrand and F. Namvar et al., 2014. Acute toxicity study of zerumbone-loaded nanostructured lipid carrier on BALB/c mice model. BioMed Res. Int., 2014.
- Humpel, N. and S.C. Jones, 2004. "I don't really know, so it's a guess": Women's reasons for breast cancer risk estimation. Asian Pac. J. Cancer Prev., 5: 428-432.
- Shimoda, H., S.J. Shan, J. Tanaka, A. Seki and J.W. Seo et al., 2010. Anti-Inflammatory properties of red ginger (Zingiber officinale var. Rubra) extract and suppression of nitric oxide production by its constituents. J. Med. Food, 13: 156-162.
- Chakraborty, D., K. Bishayee, S. Ghosh, R. Biswas, S.K. Mandal and A.R. Khuda-Bukhsh, 2012. [6]-Gingerol induces caspase 3 dependent apoptosis and autophagy in cancer cells: Drug-DNA interaction and expression of certain signal genes in HeLa cells. Eur. J. Pharmacol., 694: 20-29.
- Lee, D.Y., H. Li, H.J. Lim, H.J. Lee, R. Jeon and J.H. Ryu, 2012. Anti-inflammatory activity of sulfur-containing compounds from garlic. J. Med. Food, 15: 992-999.
- Daina, A., O. Michielin and V. Zoete, 2017. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep., 7.
- Mahady, G.B., S.L. Pendland, A. Stoia, F.A. Hamill, D. Fabricant, B.M. Dietz and L.R. Chadwick, 2005. In vitro susceptibility of Helicobacter pylori to botanical extracts used traditionally for the treatment of gastrointestinal disorders. Phytother. Res., 19: 988-991.
- Sivam, G.P., 2001. Protection against Helicobacter pylori and other bacterial infections by garlic. J. Nutr., 131: 1106S-1108S.
- Scharbert, G., M.L. Kalb, M. Duris, C. Marschalek and S.A. Kozek-Langenecker, 2007. Garlic at dietary doses does not impair platelet function. Anesth. Analg., 105: 1214-1218.
- >Gleeson, M.P., A. Hersey, D. Montanari and J. Overington, 2011. Probing the links between in vitro potency, ADMET and physicochemical parameters. Nat. Rev. Drug Discovery, 10: 197-208.
- Gunathilake, K.D.P.P. and H.P.V. Rupasinghe, 2015. Recent perspectives on the medicinal potential of ginger. Botanics: Targets Ther., 5: 55-63.
- Amagase, H., 2006. Clarifying the real bioactive constituents of garlic. J. Nutr., 136: 716S-725S.
- Fukao, H., H. Yoshida, Y.I. Tazawa and T. Hada, 2007. Antithrombotic effects of odorless garlic powder both in vitro and in vivo. Biosci. Biotechnol. Biochem., 71: 84-90.
- Mikail, H.G., 2010. Phytochemical screening, elemental analysis and acute toxicity of aqueous extract of Allium sativum L. bulbs in experimental rabbits. J. Med. Plants Res., 4: 322-326.
- Hussein, H.J., I.H. Hameed and M.Y. Hadi, 2017. A review: Anti-microbial, anti-inflammatory effect and cardiovascular effects of garlic: Allium sativum. Res. J. Pharm. Technol., 10: 4069-4078.
- Hou, T.J. and X.J. Xu, 2003. ADME evaluation in drug discovery. 3. Modeling blood-brain barrier partitioning using simple molecular descriptors. J. Chem. Inf. Comput. Sci., 43: 2137-2152.
- El-Saadony, M.T., A.M. Saad, S.A. Korma, H.M. Salem and T.A. Abd El-Mageed et al., 2024. Garlic bioactive substances and their therapeutic applications for improving human health: A comprehensive review. Front. Immunol., 15.
- Rasool, A., M.U.R. Khan, M.A. Ali, A.A. Anjum and I. Ahmed et al., 2017. Anti-avian influenza virus H9N2 activity of aqueous extracts of Zingiber officinalis (ginger) and Allium sativum (garlic) in chick embryos. Pak. J. Pharm. 30: 1341-1344.
- Ihekwereme, P.C., R.N. Asomugha, S.I. Mbagwu, D.I. Oraekei and D.L. Ajaghaku, 2023. Phytochemicals, acute toxicities and actual median lethal doses (actual LD50) of Zingiber officinale and Allium sativum given singly and in combination via mice models. GSC Biol. Pharm. Sci., 25: 8-18.
- Citronberg, J., R. Bostick, T. Ahearn, D.K. Turgeon and M.T. Ruffin et al., 2013. Effects of ginger supplementation on cell-cycle biomarkers in the normal-appearing colonic mucosa of patients at increased risk for colorectal cancer: Results from a pilot, randomized, and controlled trial. Cancer Prev. Res., 6: 271-281.
- Guo, J.J., C.M. Kuo, J.W. Hong, R.L. Chou, Y.H. Lee and T.I. Chen, 2015. The effects of garlic-supplemented diets on antibacterial activities against Photobacterium damselae subsp. piscicida and Streptococcus iniae and on growth in Cobia, Rachycentron canadum. Aquaculture, 435: 111-115.
- Funk, J.L., J.B. Frye, J.N. Oyarzo and B.N. Timmermann, 2009. Comparative effects of two gingerol-containing Zingiber officinale extracts on experimental rheumatoid arthritis. J. Nat. Prod., 72: 403-407.
- Chang, J.S., K.C. Wang, C.F. Yeh, D.E. Shieh and L.C. Chiang, 2013. Fresh ginger (Zingiber officinale) has anti-viral activity against human respiratory syncytial virus in human respiratory tract cell lines. J. Ethnopharmacol., 145: 146-151.
- Kwak, J.S., J.Y. Kim, J.E. Paek, Y.J. Lee, H.R. Kim, D.S. Park and O. Kwon, 2014. Garlic powder intake and cardiovascular risk factors: A meta-analysis of randomized controlled clinical trials. Nutr. Res. Pract., 8: 644-654.
- Kyung, K.H., 2012. Antimicrobial properties of Allium species. Curr. Opin. Biotechnol., 23: 142-147.
- Rahman, K., 2001. Historical perspective on garlic and cardiovascular disease. J. Nutr., 131: 977S-979S.
- Lawson, L.D. and S.M. Hunsaker, 2018. Allicin bioavailability and bioequivalence from garlic supplements and garlic foods. Nutrients, 10.
- Lipinski, C.A., F. Lombardo, B.W. Dominy and P.J. Feeney, 2001. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Delivery Rev., 46: 3-26.
- Nanchari, S.R., S. Perugu and V. Venkatesan, 2020. Molecular docking studies to understand the potential role of ginger compounds (6-gingerol and 6-shogaol) on anti-angiogenic and anti-lymphangiogenic mechanisms. Int. J. Chem., 12: 61-68.
- Lorke, D., 1983. A new approach to practical acute toxicity testing. Arch. Toxicol., 54: 275-287.
- Camero, M., G. Lanave, C. Catella, P. Capozza and A. Gentile et al., 2019. Virucidal activity of ginger essential oil against caprine alphaherpesvirus-1. Vet. Microbiol., 230: 150-155.
- Park, M., J. Bae and D.S. Lee, 2008. Antibacterial activity of (10)-gingerol and (12)-gingerol isolated from Ginger rhizome against periodontal bacteria. J. Phytother. Res., 22: 1446-1449.
- Iciek, M., I. Kwiecien and L. Wodek, 2009. Biological properties of garlic and garlic-derived organosulfur compounds. Environ. Mol. Mutagen., 50: 247-265.
- Cheng, F., W. Li, G. Liu and Y. Tang, 2013. In silico ADMET prediction: Recent advances, current challenges and future trends. Curr. Top. Med. Chem., 13: 1273-1289.
- Shukla, Y. and M. Singh, 2007. Cancer preventive properties of ginger: A brief review. Food Chem. Toxicol., 45: 683-690.
- Karna, P., S. Chagani, S.R. Gundala, P.C.G. Rida and G. Asif et al., 2012. Benefits of whole ginger extract in prostate cancer. Br. J. Nutr., 107: 473-484.
- Mehrbod, P., E. Amini and M.T. Kheiri, 2009. Antiviral activity of garlic extract on influenza virus. Iran. J. Virol., 3: 19-23.
- Bresso, E., V. Leroux, M. Urban, K.E. Hammond-Kosack, B. Maigret and N.F. Martins, 2016. Structure-based virtual screening of hypothetical inhibitors of the enzyme longiborneol synthase-a potential target to reduce Fusarium head blight disease. J. Mol. Model., 22.
- Grzanna, R., L. Lindmark and C.G. Frondoza, 2005. Ginger-an herbal medicinal product with broad anti-inflammatory actions. J. Med. Food., 8: 125-132.
- Nicoll, R. and M.Y. Henein, 2009. Ginger (Zingiber officinale Roscoe): A hot remedy for cardiovascular disease? Int. J. Cardiol., 131: 408-409.
- Anyanwu, R.O., A.U. Onochie and F.O. Idama, 2023. Assessment of hepatoprotective potential of ethanolic extract of Allium sativum (garlic) in diabetes induced male Wistar albino rats. J. Adv. Med. Pharm. Sci., 25: 8-13.
- Zick, S.M., Z. Djuric, M.T. Ruffin, A.J. Litzinger and D.P. Normolle et al., 2008. Pharmacokinetics of 6-gingerol, 8-gingerol, 10-gingerol, and 6-shogaol and conjugate metabolites in healthy human subjects. Cancer Epidemiol. Biomarkers Prev., 17: 1930-1936.
- Sameer Uz Zaman, M.M. Mirje and S. Ramabhimaiah, 2014. Evaluation of the anti-ulcerogenic effect of Zingiber officinale (ginger) root in rats. Int. J. Curr. Microbiol. Appl. Sci., 3: 347-354.
- Xu, S., H. Zhang, T. Liu, W. Yang and W. Lv, 2020. 6-Gingerol induces cell-cycle G1-phase arrest through AKT-GSK 3β-cyclin D1 pathway in renal-cell carcinoma. Cancer Chemother. Pharmacol., 85: 379-390.
- Dieng, S.I.M., F. Bah, A. Sarr and A.D. Fall, 2021. Pharmacological properties and toxicity of garlic and ginger: A review. Int. J. Med. Plants Nat. Prod., 7: 10-17.
- Shang, A., S.Y. Cao, X.Y. Xu, R.Y. Gan and G.Y. Tang et al., 2019. Bioactive compounds and biological functions of garlic (Allium sativum L.). Foods, 8.
- Srikanth, M., M.L. Sathyanarayana, N. Chandrashekhara and K.P. Manjunath, 2013. Biochemical studies on the effect of aged garlic extract on acetaminophen induced hepatotoxicity in rats. Indian J. Vet. Sci. Biotechnol., 9: 9-11.
- Jiang, T.A., 2019. Health benefits of culinary herbs and spices. J. AOAC Int., 102: 395-411.
- Tesfaye, A., 2021. Revealing the therapeutic uses of garlic (Allium sativum) and its potential for drug discovery. Sci. World J., 2021.
- Setiawan, V.W., G.P. Yu, Q.Y. Lu, M.L. Lu and S.Z. Yu et al., 2005. Allium vegetables and stomach cancer risk in China. Asia. Pac. J. Cancer Prev., 6: 387-395.
- van de Waterbeemd, H. and E. Gifford, 2003. ADMET in silico modelling: Towards prediction paradise? Nat. Rev. Drug Discovery, 2: 192-204.
- Veber, D.F., S.R. Johnson, H.Y. Cheng, B.R. Smith, K.W. Ward and K.D. Kopple, 2002. Molecular properties that influence the oral bioavailability of drug candidates. J. Med. Chem., 45: 2615-2623.
- Xiong, X.J., P.Q. Wang, S.J. Li, X.K. Li, Y.Q. Zhang and J. Wang, 2015. Garlic for hypertension: A systematic review and meta-analysis of randomized controlled trials. Phytomedicine, 22: 352-361.
- Temitope, O.H., A.F. Aluko, O.M. Abimbola, M.A. Abah and N.C. Emmanuella, 2025. Protective effect of Curcuma longa-honey and Allium sativum-Zingiber officinale-honey combinations on hematological parameters in rats induced with paracetamol toxicity. Am. J. Biomed. Sci. Res., 26: 744-749.
- Sivasothy, Y., W.K. Chong, Abdul Hamid, I.M. Eldeen, S.F. Sulaiman and K. Awang, 2011. Essential oils of Zingiber officinale var. rubrum Theilade and their antibacterial activities. Food Chem., 124: 514-517.
- Elengoe, A. and E. Sebestian, 2020. In silico molecular modelling and docking of allicin, epigallocatechin-3-gallate and gingerol against colon cancer cell proteins. Asia Pac. J. Mol. Biol. Biotechnol., 28: 51-67.
- Al-Amin, Z.M., M. Thomson, K.K. Al-Qattan, R. Peltonen-Shalaby and M. Ali, 2006. Anti-diabetic and hypolipidaemic properties of ginger (Zingiber officinale) in streptozotocin-induced diabetic rats. Br. J. Nutr., 96: 660-666.
- Chinonso, A.D., A.A. Kayode, M.A. Adondua and U.K. Chinekwu, 2025. Biochemistry of traditional herbal compounds and their molecular targets. Pharmacogn. Rev., 19: 83-90.
- Ale, E.M., I.J. Kade, M.J. Timothy, R.H.N. Boyi and S.O. Asuelimen et al., 2025. Assessment of the participation of sulfhydryl proteins in the glutathione peroxidase mimicry of diphenyl diselenide in the presence of thiol alkylating agent. Sci. Rep., 15.
- Oyibol, O.N., M.U. Attah, G.C. Ezeah, E.C. Mgbeokwere and A.S. Okabeonye et al., 2025. Evaluation of the antimalarial and hepatoprotective effects of Jatropha curcas leaf extract in Plasmodium berghei-infected mice. Am. J. Biomed. Sci. Res., 26: 750-756.
- Unekwuojo, A.G., O.A. Francis, I.E. Peter, A.A. Rapheal and O.M. Abimbola et al., 2025. Histopathological assessment of kidney microstructure following exposure to gentamicin and Annona muricata seed oil in albino rats. JOJ Urol. Nephrol., 9.
How to Cite this paper?
APA-7 Style
Oladosu,
M.A., Abah,
M.A., Aghworo,
E.M., Justina,
O.D., Oluwaseyi,
A.P., Farinde,
T.D., Farinde,
O.N., Osaro,
N.O., Ighodaro,
F.U., Onyeoche,
A.S., Kennedy,
Z. (2025). Toxicity Assessment of Allium sativa and Zingiber officinale using in vivo and in silico Approaches in Mice. Trends in Pharmacology and Toxicology, 1(1), 1-11. https://doi.org/10.21124/tpt.2025.1.11
ACS Style
Oladosu,
M.A.; Abah,
M.A.; Aghworo,
E.M.; Justina,
O.D.; Oluwaseyi,
A.P.; Farinde,
T.D.; Farinde,
O.N.; Osaro,
N.O.; Ighodaro,
F.U.; Onyeoche,
A.S.; Kennedy,
Z. Toxicity Assessment of Allium sativa and Zingiber officinale using in vivo and in silico Approaches in Mice. Trends Pharm. Toxicol. 2025, 1, 1-11. https://doi.org/10.21124/tpt.2025.1.11
AMA Style
Oladosu
MA, Abah
MA, Aghworo
EM, Justina
OD, Oluwaseyi
AP, Farinde
TD, Farinde
ON, Osaro
NO, Ighodaro
FU, Onyeoche
AS, Kennedy
Z. Toxicity Assessment of Allium sativa and Zingiber officinale using in vivo and in silico Approaches in Mice. Trends in Pharmacology and Toxicology. 2025; 1(1): 1-11. https://doi.org/10.21124/tpt.2025.1.11
Chicago/Turabian Style
Oladosu, Micheal, Abimbola, Moses Adondua Abah, Eloho Mathilda Aghworo, Omotosho Damilola Justina, Adeyeye Pius Oluwaseyi, Tobi David Farinde, Olutayo Nathanael Farinde, Nosakhare Odionfo Osaro, Faith Ukamaka Ighodaro, Abah Sarah Onyeoche, and Zion Kennedy.
2025. "Toxicity Assessment of Allium sativa and Zingiber officinale using in vivo and in silico Approaches in Mice" Trends in Pharmacology and Toxicology 1, no. 1: 1-11. https://doi.org/10.21124/tpt.2025.1.11

This work is licensed under a Creative Commons Attribution 4.0 International License.




